Issue № 6 — 07/10/2022 Биология
MICRONEEDLES: A NEW APPROACH TO DRUG DELIVERY (Review)
This is an automatic translation.
Click here to read the publication in the original language.
Summary
The review describes a new technology for the delivery of drugs, drugs based on peptides, vaccines, hormones using microneedles, with the main attention being paid to the use of soluble microneedles. Microneedles have emerged as an alternative method of transdermal drug delivery and have found application in many areas of biomedicine, such as therapy, diagnostics, and vaccine administration. The development of technology for the production and use of microneedles continues, their quality is constantly improved due to the use of inert or biosoluble materials, as well as such advantages as less discomfort during application, the possibility of self-administration of drugs, increased stability, convenience and safety of use. The review also outlines the prospects for the development of this technology.
Key words : microneedles, transdermal administration, vaccines, microcasting, microneedle platform, soluble microneedles.
Introduction
Microneedles are a recently developed drug delivery system through which pharmaceutical and cosmetic substances (proteins, polysaccharides, nucleic acids, hormones, vaccines, etc.) are introduced into the skin in a minimally invasive way. Percutaneous administration of drugs or vaccines is usually associated with painful procedures and a negative psychological effect, which is most pronounced in children, but is present to some extent in adults, therefore, methods with a less pronounced pain effect and a reduced sense of fear in the patient are increasingly application including in preventive medicine. In addition, damage to the skin by injection needles can lead to infection and the development of post-injection complications. If asepsis rules are violated, as well as when non-sterile solutions are used, an infiltrate or abscess is formed. In immunosuppressed patients, a systemic inflammatory response and sepsis may develop, infection with hepatitis B and C, and HIV infection is possible. A separate aspect of this problem is the self-injury of medical workers with a non-sterile needle. The World Health Organization has found that 37% of cases of hepatitis B and 4.4% of HIV occur when the skin is injured, and medical workers are at risk of contracting these blood-borne infections [1]. The development of non-injection methods for the administration of drugs is the most important problem in medicine.
One of the solutions to this problem was a platform carrying on the surface an array of microneedles with coated or encapsulated drug in microneedles (MI). They are being studied by many researchers to overcome the limitations of traditional approaches. MI can be designed in such a way that the depth of penetration into the skin depends on the purpose of the introduction from quite superficial to deep. In the first case, the microneedles will not touch the nerve receptors, which usually leads to painful injection of drugs. MI is an array on a substrate with an adhesive protective layer that forms a patch for application to the skin [2]. MI platforms are typically less than a millimeter high. The present review considers the ways of development and prospects for the use of soluble microneedles for the delivery of drugs, drugs based on peptides, vaccines, and hormones. The concept of a microneedle platform originated in the late 1970s, but technologically then it could not develop quickly. Due to the development of nanotechnology in the 1990s. MI has received increasing attention and new perspectives have been opened up in clinical medicine [3]. Since MIs do not penetrate deeper than the protective layer of the skin, the likelihood of infection is much less. Thus, the use of microneedles is safer than conventional subcutaneous injection [4].
Transdermal administration of drugs
The skin is the largest external organ of the human body. The skin area occupies about 1.8 sq.m, by weight it is approximately one fifth of the total body weight [5]. The skin is a barrier against a wide range of external influences and protects against the penetration of pathogens, toxins into the body, as well as from the harmful effects of ultraviolet radiation. It is involved in the processes of thermoregulation, respiration and many others. Because of these qualities, the skin can be considered as a critical area for a selective and non-invasive drug delivery route. A transdermal therapeutic system (TTS) is a dosage soft dosage form for external use in the form of patches or films that releases the drug over a certain time [6]. With transdermal drug administration, a number of issues associated with oral administration are removed, such as gastric irritation, better drug release over time compared to oral administration [7]. Ease of use, painlessness, safety, no need for frequent use are the positive qualities of TTS. Examples of drugs that are administered with TTS are scopalamin (Transderm-Scop), fentanyl (Lexicomp-Online), nicotine (Nicoderm) [7], diclofenac, lidocaine, nitroglycerin, estradiol, etc. [6]. With transdermal administration, there are a number of limitations, primarily related to the need to overcome the stratum corneum of the skin, which acts as the first protective layer and limits the absorption of the drug. For transdermal administration, a number of requirements are imposed on the drug: the molecular weight of the substance should not exceed 600 Da, good permeability through the skin, low melting point, good solubility, and no charge on the substance molecule. Therefore, not all drugs meet these requirements. At the first stage of development of TTS, plasters were used, which are still used in wide practice. The number of drugs in this case is low, and they should fit the range of molecular weight, hydrophilicity and efficiency at low dosage. The advantages of this form include ease of use and a fairly low cost of manufacture. The next stage in the development of TTS was the use of the methods of iontophoresis, sonophoresis, activators of the transfer of medicinal substances. These methods make it possible to increase the dose of penetrating substances, and, accordingly, the effectiveness of the impact, but require the use of devices and medical personnel for practical application. The third step was new drug transfer activators, MI, which allowed large molecules to better penetrate the outer layer of the skin [7]. In recent years, microneedle technology has developed rapidly, allowing the use of drugs with a large molecular weight compared to other methods of the transdermal therapeutic system. Through microchannels created by microneedles in the outer layer of the skin, medicinal substances penetrate into the outer stratum corneum of the skin. At the same time, they do not affect the nerve endings in the skin, so the pain effect of the procedure is minimized or completely absent. Microneedles can provide transdermal delivery of proteins, peptides, antibodies, vaccines, and drugs with poor efficacy at low doses. Also, patients can self-administer the drug. With the development of nanotechnology, many pharmaceutical companies have been involved in the development of microneedles [8]. MI is divided into the following categories: solid, hollow, coated and soluble. Solid MIs are made from inert materials such as stainless steel, titanium, and silicon. Their role: to make channels in the skin through which the applied drug penetrates beyond the stratum corneum. Hollow MPs are made from the same inert materials as solid MPs. Hollow MIs are designed to have hollow channels within each needle that allow drug delivery through holes into the skin in a manner similar to conventional needles, but without the drawbacks mentioned. Coated MIs are similar to solid MIs and are made of inert materials such as stainless steel or titanium, coated with the desired compound by dipping or spraying the composition onto the MI. Soluble MPs differ from the previous three categories in that they are made from materials that dissolve after being injected into the skin [2].
Types, varieties, materials for the manufacture of microneedles
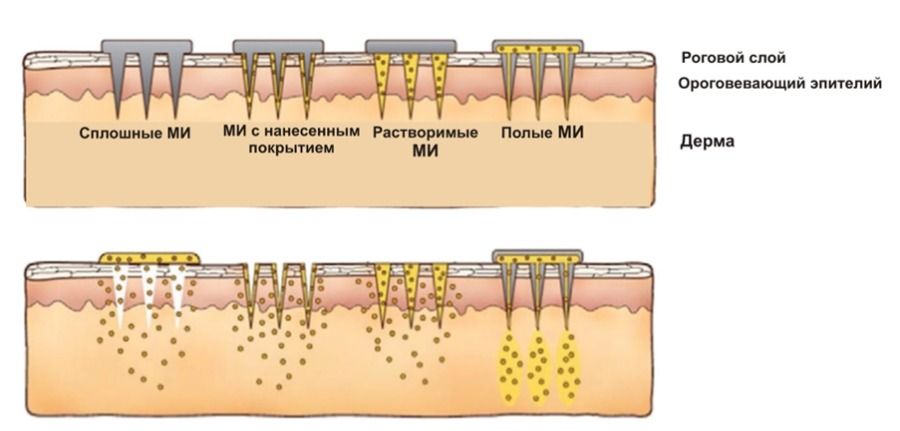
MI is a plate, on the surface of which there is an array of 10 to 1000 microneedles. Microneedle sizes range from 100 to 1000 µm. MI can be divided into four main groups: solid, hollow, coated and soluble (Figure 1) [8]. Soluble MIs can be made from a polymer with drug substance (or substances) distributed evenly throughout the entire volume of the needle. There are also multilayer MIs, where the needle consists of several layers of polymer with different drugs. In appearance, MI are divided into conical, pyramidal, teardrop-shaped and complex shapes.
Figure 1 - Varieties of microneedles
Solid MI
Solid or solid state MIs were developed in 1998. They are used to pretreat skin to create microchannels that increase skin permeability. Then, a patch with a medicinal substance or other topical dosage form, for example, a gel, ointment, cream, solution, is applied over these channels. Further, the medicinal substance from the patch or other dosage form penetrates through these microchannels. As a result, the introduction of drugs such as captopril and metoprolol tartrate through the stratum corneum is improved [9]. Solid MIs are easy to manufacture and have increased mechanical strength. They are made of silicon, glass, titanium, stainless steel, etc. The process of deep reactive ion etching is used to produce silicon microneedles [8]. MIs made of stainless steel are manufactured using micro-milling technology, laser cutting, and photolithographic wet etching [8]. Solid MIs can also be produced from polymers by microcasting and 3D printing [10].
coated MI
Coated MIs are the same solid MIs that are coated with a medicinal substance during the manufacturing process. Many methods have been described for the manufacture of coated microneedles [8]. The coating on the MI is carried out by immersing the microneedles in the solution or spraying the solution onto the needles. One of the methods for coating MIs is the use of electrohydrodynamic sputtering [11]. Lithography methods were used to apply a pattern on the foil, imitating the structure of the skin, followed by applying the foil to the MI [12]. These MIs have advantages over solid MIs, such as the absence of an immune system response to MIs themselves, and virtually painless administration, which makes it possible to use MIs on more sensitive areas of the skin. After piercing the skin with coated microneedles, the drug dissolves in the skin and diffuses into the microcirculation system. After insertion, the microneedles can be removed [11]. In addition to drug administration, MIs can also be used as electrodes for measuring the electrical characteristics of the skin and corresponding organs [13].
Hollow MI
Hollow MIs consist of a drug reservoir and a hollow hole in the center of the needle. The introduction of a drug substance is carried out by controlling the pressure or electricity of the flow of a drug solution using a pump, syringe or pressurized gas [10]. Hollow MIs also pass through the stratum corneum, and the drug enters the lower layers of the epidermis. In the manufacture of hollow microneedles, the methods of isotropic etching [11], laser micromachining [8], complex lithographic casting by the method of liquid chemical etching and X-ray photolithography [8] are used. The following materials are used to manufacture hollow microneedles: silicon single crystal [9], stainless steel [8], polylactic acid [8], carbon nanotubes [8], poly(methyl methacrylate) [8], glass and ceramics [10]. Hollow MIs are used to administer macromolecular substances such as proteins, oligonucleotides, and vaccines. They are also used for transdermal delivery of insulin [8, 9] and biomedical diagnostics [4].
Soluble MI
Soluble MIs are designed in such a way that after administration they completely dissolve in the skin without leaving any residue. Soluble MIs work on a "press and release" basis. In the production of microneedles, biocompatible and water-soluble materials are used, which are mixed with the medicinal substance. Unlike other types of microneedles, soluble MIs dissolve in the skin after penetrating the skin, releasing the drug substance. They have a number of advantages: simplified manufacturing, ease of use, high drug load [14]. In the manufacture of microneedles, microforms are usually used, which are filled with a solution of a polymer with a medicinal substance [15]. The manufacturing process consists of pouring the polymer solution into micro molds, filling the mold micro cavities by centrifugation, vacuum or pressure, drying at room temperature and pressure. Various substances are used as the basis for soluble microneedles: silicone [16], polyvinyl alcohol [2, 19, 21], polyvinylpyrrolidone [17, 19], carboxymethyl cellulose [2, 21], hyaluronic acid [18, 21], polyvinyl acetate [2 ], polylactic acid [2, 14], gelatin [2], chondroitin sulfate [2, 21, 22], sorbitol [2, 20], sucrose [2, 22], trehalose [2, 19, 22], dextran 70 [20], polyacrylamide [2], polyacrylic acid [22], etc. Soluble MI can be conical, pyramidal, and complex in shape. Soluble can be part of the needle, for example, only the tip [23]. The soluble part can be located on a solid insoluble base [23]. After administration, the soluble part remains in the skin, while the solid base is separated and removed. With the help of two-photon polymerization, it is possible to create more complex shapes of microneedles [24]. Such modifications allow rapid drug delivery and controlled release.
Use of microneedles
MI is increasingly playing an important role in medicine and cosmetology. The first microneedling product to enter the market was the dermaroller [23]. Soluble MIs with hyaluronic acid are widely used in cosmetology. All these products have appeared on the market recently and are becoming increasingly popular [15].
Dosage forms developed using microneedles
The introduction of lidocaine using microneedles is more comfortable and more effective compared to subcutaneous administration [23]. Soluble MIs with meloxicam were used to treat neuropathic pain [23]. Chemotherapeutic methods of cancer treatment have been developed using microneedles for the administration of various anticancer drugs, such as cisplatin and doxorubicin [25], 5-fluorouracil, tamoxifen, and gemcitabine [23]. Localized administration of these drugs helps reduce side effects. MI with insulin [8, 9, 25, 26] and exendin-4 [26] were used to treat diabetes. MI with parathyroid hormone is used to treat osteoporosis [26]. Patches with microneedles filled with drugs such as levonorgestrel, progesterone, etonogestrel as hormonal contraception have been developed [26]. MI with antiretroviral drugs (rilpivirine, acyclovir, lamivudine) improved the stability of the drugs, increased the degree of penetration through the skin and increased the parameters of local distribution [25].
Transdermal administration of high molecular weight drugs is limited due to the barrier properties of the stratum corneum. MI helps to solve this problem - the effectiveness of the method of treating chronic thromboembolism using microneedles with heparin has been shown [27]. Studies on the introduction of peptides using microneedles have shown that soluble MIs are able to deliver peptides through the stratum corneum of the skin. The stability of peptides is also preserved at the stages of development, application, and delivery [28].
Development of microneedle-based vaccines
Transdermal administration of vaccines using dissolvable microneedles is a rapidly growing area of research. The introduction of vaccines using microneedles has advantages over intramuscular and subcutaneous administration. Since MIs allow the vaccine to be delivered to the epidermis and dermal region, which contains many Langerhans cells and dendritic cells, the vaccine elicits a more pronounced immune response with this route of administration.
The use of MI technology for vaccination has a number of advantages. This method of vaccine administration does not require the involvement of highly qualified personnel, which can be useful, for example, during mass vaccinations [30]. The use of sterile-packed medical devices with vaccine applied reduces the risk of post-injection complications and eliminates the risk of infection with hepatitis and HIV through self-traumatization by non-sterile needles in medical staff.
Studies on the use of microneedles for influenza vaccination have shown the production of strong antibodies and cellular immune responses in mice, providing complete protection against lethal infection [31]. Human studies have shown that influenza vaccination using microneedles is well tolerated and elicits a strong immunological response (Figure 2) [32].
Microneedle patches developed for the administration of inactivated polio vaccine have shown better thermal stability during long-term storage at elevated temperatures compared to conventional liquid vaccine [33]. The stability of vaccines in microneedles at room temperature was also reported in [34]. The paper presents the VaxiPatch system, which consists of subunit glycoprotein vaccine antigens, adjuvants and a transdermal delivery system. MIs with various vaccines have passed preclinical and clinical trials. Improved stability, safety and immunological efficacy have been shown compared to conventional vaccine administration [35]. The use of microneedles with ovalbumin as a model antigen for obtaining an immune response indicates the use of microneedles in the future for the introduction of antigens [36]. The development of microneedles with lyophilic influenza vaccine remained active during manufacture and subsequent storage for 3 months at 40°C and induced a strong immune response in a mouse model [37]. The use of microneedles for vaccination improves logistics and improves stability. This was shown in the work on the example of a patch with microneedles of a two-component vaccine against measles and rubella [38]. In addition to logistics and stability, MI allows for a controlled release of a vaccine over time. Thus, the need for multiple injections is reduced [39]. Over the past 10 years, interest in the use of microneedles for vaccination has greatly increased, many scientific papers have been written, patents have been obtained, and research is underway [40]. Currently, MIs with vaccines are at various stages of clinical trials [41].
Conclusion
Thus, the use of microneedle technology is a promising way to safely and effectively administer drugs both in the hospital and outpatient. The simplicity and low pain impact of MI application allows to increase the coverage of the population with vaccination. The use of a microneedle platform for immunization may be more effective than conventional intramuscular injection. MI allow solving such issues as ensuring thermal stability during storage and transportation. The most important advantages of the technology are the reduction in the dose of the active substance, ease of use, the absence of sharp objects and the ease of their further disposal, minimal pain or their complete absence during application. With the modern development of technology, there are all the necessary components to create a domestic production of microneedles. Thanks to continued research work, the use of MIs will play a leading role in immunization and the treatment of chronic noncommunicable diseases in the next decade.
List of sources used
- Riddell A, Kennedy I, Tong CY. Management of sharps injuries in the healthcare setting. //BMJ. 2015 Jul 29;351:h3733. doi: 10.1136/bmj.h3733. PMID: 26223519.
- Moore LE, Vucen S, Moore AC. Trends in drug- and vaccine-based dissolvable microneedle materials and methods of fabrication.// Eur J Pharm Biopharm. 2022 Apr;173:54-72. doi: 10.1016/j.ejpb.2022.02.013. Epub 2022 Feb 25. PMID: 35219862.
- Li J, Zeng M, Shan H, Tong C. Microneedle Patches as Drug and Vaccine Delivery Platform.// Curr Med Chem. 2017;24(22):2413-2422. doi: 10.2174/0929867324666170526124053. PMID: 28552053.
- Romanyuk A.V., Zvezdin V.N., Samant P., Zemlyanova M.A., Prauznitz M.R., Ustinova O.Yu. Development of microneedle applicators for biomedical diagnostics // Fundamental research. - 2013. - No. 12-2. - S. 319-326.
- Marc B. Brown, Adrian C. Williams. The Art and Science of Dermal Formulation Development.// 2019. doi.org/10.1201/9780429059872.
- Kuznetsova E.G., Ryzhikova V.A., Salomatina L.A., Sevastyanov V.I. Transdermal transfer of medicinal substances and ways to enhance it.// Bulletin of transplantology and artificial organs. 2016;18(2):152-162. doi.org/10.15825/1995-1191-2016-2-152-162.
- Ahmed Saeed Al-Japairai K, Mahmood S, Hamed Almurisi S, Reddy Venugopal J, Rebhi Hilles A, Azmana M, Raman S. Current trends in polymer microneedle for transdermal drug delivery.// Int J Pharm. 2020 Sep 25;587:119673. doi: 10.1016/j.ijpharm.2020.119673. Epub 2020 Jul 30. PMID: 32739388.
- Ita K. Transdermal Delivery of Drugs with Microneedles-Potential and Challenges. // Pharmaceutics. 2015 Jun 29;7(3):90-105. doi: 10.3390/pharmaceutics7030090. PMID: 26131647.
- Gupta J, Gupta R, Vanshita. Microneedle Technology: An Insight into Recent Advancements and Future Trends in Drug and Vaccine Delivery. Assay Drug Dev Technol. // 2021 Feb-Mar;19(2):97-114. doi: 10.1089/adt.2020.1022. Epub 2020 Dec 9. PMID: 33297823.
- Sirbubalo M, Tucak A, Muhamedagic K, Hindija L, Rahić O, Hadžiabdić J, Cekic A, Begic-Hajdarevic D, Cohodar Husic M, Dervišević A, Vranić E. 3D Printing-A "Touch-Button" Approach to Manufacture Microneedles for Transdermal Drug Delivery.//Pharmaceutics. 2021 Jun 22;13(7):924. doi: 10.3390/pharmaceutics13070924. PMID: 34206285.
- , &ND. S. (2019). Review of Microneedle based Transdermal Drug Delivery Systems.// International Journal of Pharmaceutical Sciences and Nanotechnology, 12(3), 4511-4523. https://doi.org/10.37285/ijpsn.2019.12.3.1.
- Jiawook R.N. Manufacturing nanoimprint lithography system to produce efficient microneedles patch for transdermal drug delivery.// J Appl Biotechnol Bioeng. 2017;3(3):321-324. doi: 10.15406/jabb.2017.03.00065.
- Miyako Arai, Yuya Nishinaka, and Norihisa Miki. Electroencephalogram measurement using polymer-based dry microneedle electrode.// 2015 Jpn. J. Appl. Phys. 54.
- He X, Sun J, Zhuang J, Xu H, Liu Y, Wu D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects.// Dose-Response. October 2019. doi:10.1177/1559325819878585.
- Manoj, VR, and H. Manoj. Review on transdermal microneedle-based drug delivery.// Asian Journal of Pharmaceutical and Clinical Research, vol. 12, no. 1, Jan. 2019, pp. 18-29, doi:10.22159/ajpcr.2019.v12i1.27434.
- Jing Ji et al. Microfabricated Silicon Microneedle Array for Transdermal Drug Delivery. // 2006 J. Phys.: Conf. Ser. 34 1127.
- S. Shim et al. Role of Polyvinylpyrrolidone in Dissolving Microneedle for Efficient Transdermal Drug Delivery: In Vitro and Clinical Studies. // Bull. Korean Chem. soc. 2018 June, Volume 39, Issue 6 Pages 789-793. doi.org/10.1002/bkcs.11476.
- Zvezdin V, Peno-Mazzarino L, Radionov N, Kasatkina T, Kasatkin I. Microneedle patch based on dissolving, detachable microneedle technology for improved skin quality - Part 1: ex vivo safety evaluation. // Int J Cosmet Sci. 2020 Aug;42(4):369-376.
doi: 10.1111/ics.12627. Epub 2020 Jul 5. PMID: 32412648. - Microneedles and methods for their manufacture. RU 2 719 937 C1;
- An array of microstructures for the delivery of acting agents. RU 2 662 432 C2;
- Microneedle applicator and method for its manufacture. RU 2 652 567 C1;
- Leone M, Mönkäre J, Bouwstra JA, Kersten G. Dissolving Microneedle Patches for Dermal Vaccination. // Pharm Res. 2017 Nov;34(11):2223-2240. doi: 10.1007/s11095-017-2223-2. Epub 2017 Jul 17. PMID: 28718050/
- Waghule et al. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. // 2019 Biomedicine & Pharmacotherapy 109, 1249-1258. doi.org/10.1016/j.biopha.2018.10.078.
- Faraji Rad, Z., Prewett, PD & Davies, GJ High-resolution two-photon polymerization: the most versatile technique for the fabrication of microneedle arrays. // 2021 Microsyst Nanoeng 7 , doi.org/10.1038/s41378-021-00298-3.
- Mdanda S, Ubanako P, Kondiah PPD, Kumar P, Choonara YE. Recent Advances in Microneedle Platforms for Transdermal Drug Delivery Technologies. // Polymers (Basel). 2021 Jul 22;13(15):2405. doi: 10.3390/polym13152405. PMID: 34372008.
- Nguyen TT, Nguyen TTD, Tran NM, Vo GV. Advances of microneedles in hormone delivery. // Biomed Pharmacother. Jan 2022;145:112393. doi: 10.1016/j.biopha.2021.112393. Epub 2021 Nov 10. PMID: 34773762.
- Arshad MS, Zafar S, Zahra AT, Zaman MH, Akhtar A, Kucuk I, Farhan M, Chang MW, Ahmad Z. Fabrication and characterization of self-applicating heparin sodium microneedle patches. // JDrugTarget. 2021 Jan;29(1):60-68. doi: 10.1080/1061186X.2020.1795180. Epub 2020 Jul 27. PMID: 32649227.
- Dillon C, Hughes H, O'Reilly NJ, Allender CJ, Barrow DA, McLoughlin P. Dissolving microneedle based transdermal delivery of therapeutic analogue peptides. // Int J Pharm. 2019 Jun 30;565:9-19. doi: 10.1016/j.ijpharm.2019.04.075. Epub 2019 Apr 29.
PMID: 31047995; - Menon I, Bagwe P, Gomes KB, Bajaj L, Gala R, Uddin MN, D'Souza MJ, Zughaier SM. Microneedles: A New Generation Vaccine Delivery System. // Micromachines (Basel). 2021 Apr 14;12(4):435. doi: 10.3390/mi12040435. PMID: 33919925.
- Rodgers AM, Cordeiro AS, Donnelly RF. Technology update: dissolvable microneedle patches for vaccine delivery. // Med Devices (Auckl). 2019 Sep 19;12:379-398. doi: 10.2147/MDER.S198220. PMID: 31572025.
- Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. Dissolving polymer microneedle patches for influenza vaccination. // Nat Med. 2010 Aug;16(😎:915-20. doi: 10.1038/nm.2182. Epub 2010 Jul 18. PMID: 20639891.
- Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR ; TIV-MNP 2015 Study Group. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. // Lancet. 2017 Aug 12;390(10095):649-658. doi: 10.1016/S0140-6736(17)30575-5. Epub 2017 Jun 27. PMID: 28666680.
- Kolluru C, Gomaa Y, Prausnitz MR. Development of a thermostable microneedle patch for polio vaccination. // Drug Deliv Transl Res. 2019 Feb;9(1):192-203. doi: 10.1007/s13346-018-00608-9. PMID: 30542944.
- Thomas J. Ellison, George C. Talbott, Daniel R. Henderson. VaxiPatchTM, a novel vaccination system comprised of subunit antigens, adjuvants and microneedle skin delivery: An application to influenza B/Colorado/06/2017. // 2020 Vaccine 38 6839-6848. doi.org/10.1016/j.vaccine.2020.07.040.
- T. Nguyen et al. Progress in microneedle array patch (MAP) for vaccine delivery. // 2021 Human Vaccines & Immunotherapeutics, 17(1), 316 - 327.
- Lee SJ, Lee HS, Hwang YH, Kim JJ, Kang KY, Kim SJ, Kim HK, Kim JD, Jeong DH, Paik MJ, Yee ST. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin. PLOS One. 2019 Aug 6;14(😎:e0220382.
doi: 10.1371/journal.pone.0220382. PMID: 31386690. - Kim YC, Lee JW, Esser ES, Kalluri H, Joyce JC, Compans RW, Skountzou I, Prausnitz MR. Fabrication of microneedle patches with lyophilized influenza vaccine suspended in organic solvent. Drug Deliv Transl Res. 2021 Apr;11(2):692-701. doi: 10.1007/s13346-021-00927-4. Epub 2021 Feb 15. PMID: 33590465.
- Joyce JC, Collins ML, Rota PA, Prausnitz MR. Thermostability of Measles and Rubella Vaccines in a Microneedle Patch. Adv Ther (Weinh). 2021 Oct;4(10):2100095. doi: 10.1002/adtp.202100095. Epub 2021 Jul 28. PMID: 34926791.
- Mazzara JM, Ochyl LJ, Hong JKY, Moon JJ, Prausnitz MR, Schwendeman SP. Self-healing encapsulation and controlled release of vaccine antigens from PLGA microparticles delivered by microneedle patches. Bioeng Transl Med. 2018 Oct 30;4(1):116-128. doi: 10.1002/btm2.10103. PMID: 30680323.
- Ingrole RSJ, Azizoglu E, Dul M, Birchall JC, Gill HS, Prausnitz MR. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. biomaterials. Jan 2021;267:120491. doi: 10.1016/j.biomaterials.2020.120491. Epub 2020 Nov 5. PMID: 33217629.
- Zheng Z, Diaz-Arévalo D, Guan H, Zeng M. Noninvasive vaccination against infectious diseases. Hum Vaccine Immunother. 2018 Jul 3;14(7):1717-1733. doi: 10.1080/21645515.2018.1461296. Epub 2018 May 17. PMID: 29624470.
Download article in PDF (438 KB)
This is an automatic translation.
Click here to read the publication in the original language.